510k submission
510k Submission Process
The 510k submission is the second most common pathway for marketing a medical device in the United States. It demonstrates that the device is substantially equivalent to a legally marketed predicate device that is not subject to premarket approval. A 510k Submission document preparation including device testing, and clinical data consolidation takes up almost 4 to 5 months. Post submissions to the FDA generally take up to 6 months, for closing the review queries.
Our 510k consultants’ careful plan, strategic decisions, and expertise will ensure the early acceptance of the 510k file without RTA or AI and the successful 510k clearance of the submission. All applicants must submit a soft copy on CD and an e-copy. All foreign manufacturers are requested to have a US Agent for FDA correspondence officially.
Note: The service of the US Agent is different from the US Agent service for Establishment Registration.
Pre and Q 510k Submission
Pre or Q 510k Submission are the two types of submissions that allow the manufacturer/applicant to request formal feedback on files specific to (a) Test Protocols, (b) Substantial Equivalence, (c) Missing Sections, etc.
It allows the manufacturer/applicant to request formal feedback on the files of your medical device, specifically on (a) test protocols (b) substantial equivalence, (c) missing sections, etc. before you make a review payment and submit an application.
Would you like us to send an email with important information about 510(k) service within the next 2 minutes?
Benefits of Pre 510k Submission
Although Pre-Submission / Q 510k submission is not mandatory, we often recommend customers do so to gain an FDA view of the documents and if you have specific questions to the FDA to clarify. The consultants use this provision to seek valuable feedback on various topics, such as costly bench and animal testing, and clinical trials.
- Better clarity on subject device requirements
- Improved quality of final 510k file,
- Enhanced transparency of the review process,
- Smooth and clear review comments,
- Potentially shorter total review times,
- No fee
We offer the best guidance and solutions for 510k submission to worldwide manufacturers and specification developers. Our services are time-bound and economical. We match prices. So partner with us and experience the difference firsthand.
510k Submission Timeline
The duration of the 510k submission process and timeline can vary significantly depending on factors such as the complexity of the device, the completeness of the submission, and any interactions required with the FDA. Generally, the Food and Drug Administration aims to complete the review process within 90 days of acceptance, but actual timelines may be longer in the majority of the cases of applicants.
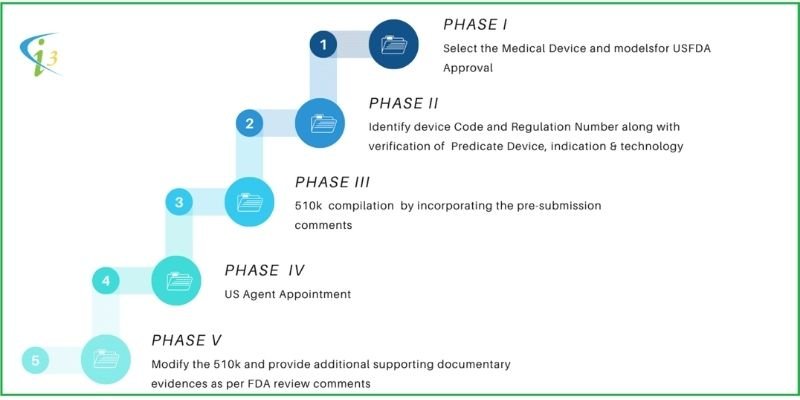
PHASE I | Stages | Activity | Responsibility | Timeline |
1 | Select the Device and models for US FDA 510k approval | CLIENT | 20 Days | |
2 | Identify Predicate Device with the same indication and technology | CLIENT + I3C | ||
3 | If NOT substantially equivalent, follow the PMA route or, if substantially equivalent, follow the 510k route | CLIENT + I3C | ||
4 | Appoint I3CGLOBAL as Technical Consultants and US Agent | CLIENT | ||
PHASE II | 5 | Identify Device Code and Regulation Number along with verification of Predicate Device, indication & technology. | I3C | 90 Days |
6 | Identify the device Class and guidance document | I3C | ||
7 | Biological evaluation and test requirement identification in line with the predicate device | I 3 C | ||
8 | Samples send to the Laboratory | CLIENT | ||
9 | Evaluation of equivalent device compilation | I3C | ||
10 | Drafting of 510k file in line with available FDA guidance document. | I3C | ||
11 | Review of Risk analysis, Equivalent device data, Biocompatibility Test/Safety test protocols | CLIENT + I3C | ||
12 | Review of Labels, User Manual/IFU, Shelf-life records/lifetime calculation, and pre-clinical study evidence | CLIENT + I3C | ||
13 | Pre 510k submission | CLIENT + I3C | ||
PHASE III | 14 | Compilation of 510k file by incorporating the pre-submission comments | I3C | 90 Days |
15 | Compilation of Pre-clinical and Biocompatibility and Safety testing | I3C | ||
16 | Compilation and release of the final draft | I 3 C | ||
17 | Review | I3C | ||
PHASE IV | 18 | US Agent Appointment | CLIENT | 20 Days |
19 | Review payment | CLIENT | ||
20 | eSTAR submission of file to FDA | I3C | ||
21 | Receipt of acknowledgement | CLIENT | ||
22 | Wait for the review comments | CLIENT | 90 Days | |
PHASE V | 23 | Modify the 510(k) and provide additional supporting documentary evidence as per FDA review comments | CLIENT + I3C | 60 Days |
24 | Re-submission | I3C | 10 Days | |
25 | Wait for the review comments or FDA 510k clearance letter | CLIENT | 90 Days | |
Comments
Post a Comment